Vuity Side Effects
Presbyopia does not occur in the pediatric population.
Vuity Eye Drops Prescribing Information
Medically reviewed by Drugs.com. Last updated on Nov 1, 2022.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
1 INDICATIONS AND USAGE
VUITY is indicated for the treatment of presbyopia in adults.
2 DOSAGE AND ADMINISTRATION
The recommended dosage of VUITY is one drop in each eye once daily.
If more than one topical ophthalmic product is being used, the products should be administered at least 5 minutes apart.
3 DOSAGE FORMS AND STRENGTHS
VUITY (pilocarpine hydrochloride ophthalmic solution) is provided as a 1.25% solution (12.5 mg/mL).
4 CONTRAINDICATIONS
VUITY is contraindicated in patients with known hypersensitivity to the active ingredient or to any of the excipients.
5 WARNINGS AND PRECAUTIONS
5.1 Blurred Vision
Miotics, including VUITY, may cause accommodative spasm. Patients should be advised not to drive or operate machinery if vision is not clear (e.g., blurred vision).
In addition, patients may experience temporary dim or dark vision with miotics, including VUITY. Patients should be advised to exercise caution in night driving and other hazardous activities in poor illumination.
5.2 Risk of Retinal Detachment
Rare cases of retinal detachment and retinal tear have been reported with miotics, including VUITY.
Individuals with pre-existing retinal disease are at increased risk. Therefore, examination of the retina is advised in all patients prior to the initiation of therapy.
Patients should be advised to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss.
5.3 Iritis
VUITY is not recommended to be used when iritis is present because adhesions (synechiae) may form between the iris and the lens.
5.4 Use with Contact Lenses
Contact lens wearers should be advised to remove their lenses prior to the instillation of VUITY and to wait 10 minutes after dosing before reinserting their contact lenses.
5.5 Potential for Eye Injury or Contamination
To prevent eye injury or contamination, care should be taken to avoid touching the dispensing bottle to the eye or to any other surface.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Hypersensitivity [see Contraindications ( 4 )]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VUITY was evaluated in 375 patients with presbyopia in two randomized, double-masked, vehicle-controlled studies (GEMINI 1 and GEMINI 2) of 30 days duration. The most common adverse reactions reported in >5% of patients were headache and conjunctival hyperemia. Ocular adverse reactions reported in 1-5% of patients were blurred vision, eye pain, visual impairment, eye irritation, and increased lacrimation.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of VUITY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to VUITY exposure.
Eye disorders : vitreous detachment, vitreomacular traction, retinal tear, retinal detachment.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of VUITY administration in pregnant women to inform a drug-associated risk. Oral administration of pilocarpine to pregnant rats throughout organogenesis and lactation did not produce adverse effects at clinically relevant doses.
Data
Human Data
No adequate and well-controlled trials of VUITY have been conducted in pregnant women. In a retrospective case series of 15 women with glaucoma, 4 patients used ophthalmic pilocarpine either pre-pregnancy, during pregnancy or postpartum. There were no adverse effects observed in patients or in their infants.
Animal Data
In embryofetal development studies, oral administration of pilocarpine to pregnant rats throughout organogenesis produced maternal toxicity, skeletal anomalies and reduction in fetal body weight at 90 mg/kg/day (approximately 970-fold higher than the maximum recommended human ophthalmic dose [MRHOD] of 0.015 mg/kg/day, on a mg/m 2 basis).
In a peri-/postnatal study in rats, oral administration of pilocarpine during late gestation through lactation increased stillbirths at a dose of 36 mg/kg/day (approximately 390-fold higher than the MRHOD). Decreased neonatal survival and reduced mean body weight of pups were observed at ≥18 mg/kg/day (approximately 200 times the recommended human daily dose of VUITY).
8.2 Lactation
Risk Summary
There is no information regarding the presence of pilocarpine in human milk, the effects on the breastfed infants, or the effects on milk production to inform risk of VUITY to an infant during lactation.
Pilocarpine and/or its metabolites are excreted in the milk of lactating rats. Systemic levels of pilocarpine following topical ocular administration are low [see Clinical Pharmacology ( 12.3 ) ] , and it is not known whether measurable levels of pilocarpine would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VUITY and any potential adverse effects on the breastfed child from VUITY.
Data
Animal Data
Following a single oral administration of 14 C-pilocarpine to lactating rats, the radioactivity concentrations in milk were similar to those in plasma.
8.4 Pediatric Use
Presbyopia does not occur in the pediatric population.
8.5 Geriatric Use
Clinical studies of VUITY did not include subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience with ophthalmic pilocarpine solutions have not identified overall differences in safety between elderly and younger patients.
10 OVERDOSAGE
Systemic toxicity following topical ocular administration of pilocarpine is rare, but occasionally patients who are sensitive may develop sweating and gastrointestinal overactivity. Accidental ingestion can produce sweating, salivation, nausea, tremors and slowing of the pulse and a decrease in blood pressure. In moderate overdosage, spontaneous recovery is to be expected and is aided by intravenous fluids to compensate for dehydration. For patients demonstrating severe poisoning, atropine, the pharmacologic antagonist to pilocarpine, should be used.
11 DESCRIPTION
VUITY (pilocarpine hydrochloride ophthalmic solution) 1.25% is a cholinergic muscarinic receptor agonist prepared as an isotonic, colorless, sterile ophthalmic solution containing 1.25% of pilocarpine hydrochloride. The chemical name for pilocarpine hydrochloride is (3S,4R)-3-ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]oxolan-2-one hydrochloride. Its molecular weight is 244.72 and its molecular formula is C 11 H 16 N 2 O 2 · HCl. Its structural formula is:
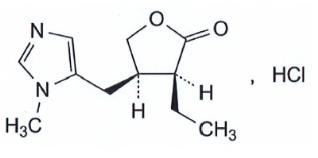
Each mL of VUITY contains pilocarpine hydrochloride 1.25% (12.5 mg) as the active ingredient, equivalent to 1.06% (10.6 mg) pilocarpine free-base. Preservative is: benzalkonium chloride 0.0075%. Inactive ingredients in the ophthalmic solution are: boric acid, sodium citrate dihydrate, sodium chloride, purified water, and may also include hydrochloric acid and/or sodium hydroxide for pH adjustment to between 3.5 and 5.5, if necessary.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pilocarpine hydrochloride is a cholinergic muscarinic agonist which activates muscarinic receptors located at smooth muscles such as the iris sphincter muscle and ciliary muscle. VUITY contracts the iris sphincter muscle, constricting the pupil to improve near and intermediate visual acuity while maintaining some pupillary response to light. VUITY also contracts the ciliary muscle and may shift the eye to a more myopic state.
12.3 Pharmacokinetics
Systemic exposure to pilocarpine was evaluated in 22 participants with presbyopia who were administered 1 drop of VUITY in each eye once daily for 30 days. The mean C max and AUC 0- t,ss values on Day 30 were 1.95 ng/mL and 4.14 ng·hr/mL, respectively. The median T max value on Day 30 was 0.3 hours postdose with a range from 0.2 to 0.5 hours postdose.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Pilocarpine did not induce tumors in mice at any dosage level studied (up to 30 mg/kg/day; approximately 160-times the MRHOD). In rats, an oral dose of 18 mg/kg/day (approximately 200 times the MRHOD), resulted in a statistically significant increase in the incidence of benign pheochromocytomas in both male and female rats, and a statistically significant increase in the incidence of hepatocellular adenomas in female rats.
Pilocarpine did not show any potential to cause genetic toxicity in a series of studies that included: 1) bacterial assays (Salmonella and E. coli) for reverse gene mutations; 2) an in vitro chromosome aberration assay in a Chinese hamster ovary cell line; 3) an in vivo chromosome aberration assay (micronucleus test) in mice; and 4) a primary DNA damage assay (unscheduled DNA synthesis) in rat hepatocyte primary cultures.
Impairment of Fertility
Pilocarpine oral administration to male and female rats at a dosage of 18 mg/kg/day (200 times the recommended human daily dose) resulted in impaired reproductive function, including reduced fertility, decreased sperm motility, and morphologic evidence of abnormal sperm. It is unclear whether the reduction in fertility was due to effects on males, females, or both. In dogs, exposure to pilocarpine at a dosage of 3 mg/kg/day for 6 months resulted in evidence of impaired spermatogenesis (approximately 110 times the recommended human daily dose).
14 CLINICAL STUDIES
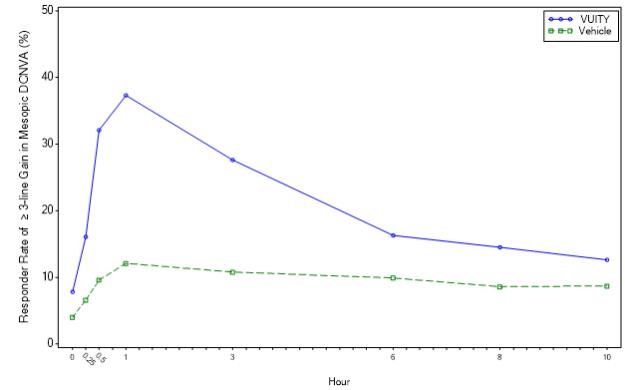
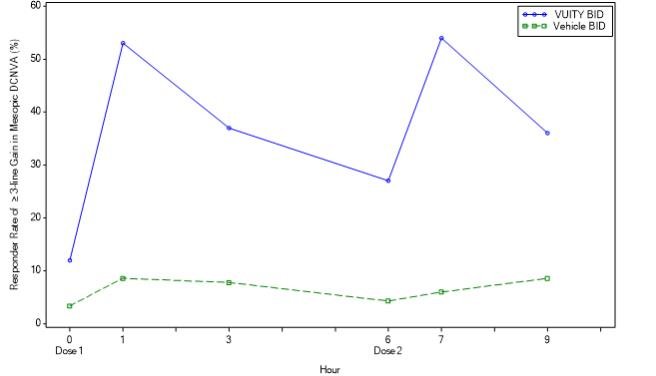
The efficacy of VUITY for the treatment of presbyopia was demonstrated in two 30‐Day Phase 3, randomized, double‐masked, vehicle‐controlled studies, namely GEMINI 1 (NCT03804268) and GEMINI 2 (NCT03857542). A total of 750 participants aged 40 to 55 years old with presbyopia were randomized (375 to VUITY group) in two studies and participants were instructed to administer one drop of VUITY or vehicle once daily in each eye.
In both studies, the proportion of participants gaining 3 lines or more in mesopic, high contrast, binocular distance corrected near visual acuity (DCNVA), without losing more than 1 line (5 letters) of corrected distance visual acuity (CDVA) with the same refractive correction was statistically significantly greater in the VUITY group compared to the vehicle group at Day 30, Hour 3 (see Table 1).
Table 1: Primary Efficacy Results from GEMINI 1 and GEMINI 2 Studies (Intent-to-Treat Population)| GEMINI 1 | GEMINI 2 | |||||
| VUITY N=163 |
Vehicle N=160 |
p-value | VUITY N=212 |
Vehicle N=215 |
p-value | |
| Proportion of participants gaining 3-lines or more in mesopic DCNVA, without losing more than 1 line (5 letters) of CDVA at Day 30, Hour 3 | 31% | 8% | p | 26% | 11% | p |
Figures 1 and 2 present the proportion of participants who gained 3-lines or more in mesopic DCNVA at Day 30.
Figure 1: Proportion of Participants Achieving 3-Lines or More Improvement in Mesopic, High Contrast, Binocular DCNVA at Day 30 in GEMINI 1 (Intent-to-Treat Population)
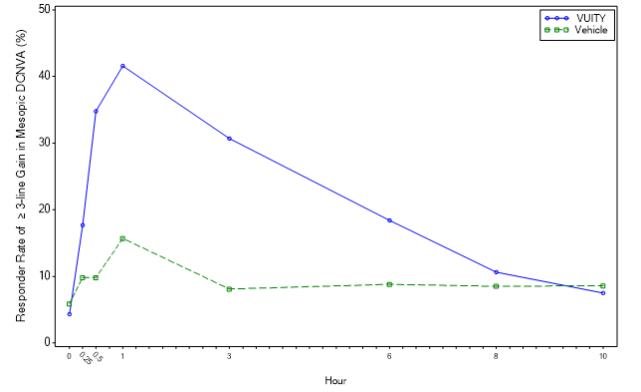
Figure 2: Proportion of Participants Achieving 3-lines or More Improvement in Mesopic, High Contrast, Binocular DCNVA at Day 30 in GEMINI 2 (Intent-to-Treat population)
How Supplied/Storage and Handling
VUITY is supplied as a sterile ophthalmic solution in colorless low density polyethylene (LDPE) ophthalmic dispenser bottles and tips, with dark green high impact polystyrene caps as follows:
| 2.5 mL fill in 5 mL bottle (Box containing 1 bottle) | NDC 0074-7098-01 |
| 2.5 mL fill in 5 mL bottle (Box containing 3 bottles) | NDC 0074-7098-03 |
| 2.5 mL fill in 5 mL bottle (Carton) | NDC 0074-7098-04 |
Store at 15°C to 25°C (59°F to 77°F). After opening, VUITY can be used until the expiration date on the bottle.
17 PATIENT COUNSELING INFORMATION
Night Driving
VUITY may cause temporary dim or dark vision. Advise patients to exercise caution with night driving and when hazardous activities are undertaken in poor illumination. [see Warnings and Precautions ( 5.1 )]
Accommodative Spasm
Temporary problems when changing focus between near and distant objects may occur. Advise patients not to drive or use machinery if vision is not clear (e.g., blurred vision). [see Warnings and Precautions ( 5.1 )]
When to Seek Physician Advice
Advise patients to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss. [see Warnings and Precautions ( 5.2 )]
Contact Lens Wear
Contact lens should be removed prior to the instillation of VUITY. Wait 10 minutes after dosing before reinserting contact lenses. [see Warnings and Precautions ( 5.4 )]
Avoiding Contamination of the Product
Do not touch dropper tip to any surface, as this may contaminate the contents. [see Warnings and Precautions ( 5.5 )]
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart.
Distributed by: Allergan, an AbbVie company
Manufactured for:
AbbVie Inc.
North Chicago, IL 60064 USA
© 2022 AbbVie. All rights reserved.
VUITY and its design are trademarks of Allergan Sales, LLC, an AbbVie company.
PRINCIPAL DISPLAY PANEL
(pilocarpine HCI ophthalmic solution) 1.25%
Contains one
2.5 mL bottle
For topical
application
in the eye
An AbbVie company

PRINCIPAL DISPLAY PANEL
(pilocarpine HCI ophthalmic solution) 1.25%
Contains three
2.5 mL bottles
For topical
application
in the eye
An AbbVie company

PRINCIPAL DISPLAY PANEL
(pilocarpine HCI ophthalmic
solution) 1.25%
For Topical Application in the Eye
An AbbVie company

PRINCIPAL DISPLAY PANEL
Professional Sample
Not for Resale
(pilocarpine HCI ophthalmic
solution) 1.25%
For Topical Application in the Eye
An AbbVie company

| Product Information | |||
| Product Type | HUMAN PRESCRIPTION DRUG LABEL | Item Code (Source) | NDC:0074-7098 |
| Route of Administration | OPHTHALMIC | DEA Schedule | |
| Active Ingredient/Active Moiety | ||
| Ingredient Name | Basis of Strength | Strength |
| Pilocarpine Hydrochloride (Pilocarpine) | Pilocarpine Hydrochloride | 12.5 mg in 1 mL |
| Inactive Ingredients | |
| Ingredient Name | Strength |
| BORIC ACID | |
| TRISODIUM CITRATE DIHYDRATE | |
| SODIUM CHLORIDE | |
| WATER | |
| HYDROCHLORIC ACID | |
| SODIUM HYDROXIDE | |
| BENZALKONIUM CHLORIDE | |
| Packaging | |||
| # | Item Code | Package Description | |
| 1 | NDC:0074-7098-01 | 1 BOTTLE in 1 BOX | |
| 1 | NDC:0074-7098-02 | 2.5 mL in 1 BOTTLE | |
| 2 | NDC:0074-7098-03 | 3 BOTTLE in 1 BOX | |
| 2 | NDC:0074-7098-02 | 2.5 mL in 1 BOTTLE | |
| 3 | NDC:0074-7098-04 | 1 BOTTLE in 1 CARTON | |
| 3 | NDC:0074-7098-02 | 2.5 mL in 1 BOTTLE | |
| 4 | NDC:0074-7098-05 | 1 BOTTLE in 1 CARTON | |
| 4 | 1.5 mL in 1 BOTTLE | ||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA214028 | 10/28/2021 | |
| Labeler – AbbVie Inc. (078458370) |
AbbVie Inc.
More about Vuity (pilocarpine ophthalmic)
- Check interactions
- Pricing & coupons
- Latest FDA alerts (1)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Breastfeeding
- En español
Patient resources
Professional resources
- Prescribing Information
- Pilocarpine Eye Drops (FDA)
Vuity Side Effects
Medically reviewed by Drugs.com. Last updated on Oct 12, 2022.
Note: This document contains side effect information about pilocarpine ophthalmic. Some dosage forms listed on this page may not apply to the brand name Vuity.
Applies to pilocarpine ophthalmic: ophthalmic solution.
Serious side effects of Vuity
Along with its needed effects, pilocarpine ophthalmic (the active ingredient contained in Vuity) may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur while taking pilocarpine ophthalmic:
More common
- Increased sweating
- muscle tremors
- nausea, vomiting, or diarrhea
- redness of the white part of the eyes or inside of the eyelids
- trouble breathing
- watering of mouth
Less common
- Blurred vision
- eye irritation or pain
- watering of the eyes
Incidence not known
- Chest tightness
- fast heartbeat
- fever
- hives, itching, skin rash
- irritation
- joint pain, stiffness, or swelling
- redness of the skin
- seeing flashes or sparks of light
- seeing floating spots before the eyes, or a veil or curtain appearing across part of vision
- swelling of the eyelids, face, lips, hands, or feet
- trouble breathing
Other side effects of Vuity
Some side effects of pilocarpine ophthalmic may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects.
Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
More common
- Decrease in night vision
- headache
For Healthcare Professionals
Applies to pilocarpine ophthalmic: compounding powder, ophthalmic gel, ophthalmic insert, ophthalmic solution.
Ocular
Common (1% to 10%): Accommodative change, blurred vision, eye irritation, visual impairment, eye pain
Frequency not reported: Ciliary spasm, itching, smarting/burning, sensitization of the lids and conjunctival vascular congestion, transient myopia, lens changes with chronic use, decrease in visual acuity in poor illumination, increased pupillary block, vitreous hemorrhaging, retinal detachment [Ref]
General
The most commonly reported side effects were accommodative change, blurred vision, eye irritation, visual impairment, and eye pain. [Ref]
Cardiovascular
Frequency not reported: Changes in blood pressure, cardiac rhythm [Ref]
Dermatologic
Frequency not reported: Sweating, increased salivation, lacrimation [Ref]
Nervous system
Common (1% to 10%): Headache, browache
Frequency not reported: Tremor [Ref]
Respiratory
Frequency not reported: Bronchial spasm, pulmonary edema [Ref]
Gastrointestinal
Frequency not reported: Nausea, vomiting, diarrhea [Ref]
More about Vuity (pilocarpine ophthalmic)
- Check interactions
- Pricing & coupons
- Latest FDA alerts (1)
- Dosage information
- During pregnancy
- FDA approval history
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
References
1. “Product Information. Pilopine-HS (pilocarpine ophthalmic).” Alcon Laboratories Inc
2. Cerner Multum, Inc. “UK Summary of Product Characteristics.” O 0
3. Cerner Multum, Inc. “Australian Product Information.” O 0
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Some side effects may not be reported. You may report them to the FDA.
Vuity Side Effects Center
Vuity (pilocarpine hydrochloride ophthalmic solution) is a cholinergic muscarinic receptor agonist indicated for the treatment of presbyopia in adults.
What Are Side Effects of Vuity?
Side effects of Vuity include:
- headache and
- eye redness.
Vuity may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- blurred vision,
- eye pain,
- visual impairment,
- eye irritation, and
- excessive tearing or watering of the eye
Get medical help right away, if you have any of the symptoms listed above.
Seek medical care or call 911 at once if you have the following serious side effects:
- Serious eye symptoms such as sudden vision loss, blurred vision, tunnel vision, eye pain or swelling, or seeing halos around lights;
- Serious heart symptoms such as fast, irregular, or pounding heartbeats; fluttering in your chest; shortness of breath; and sudden dizziness, lightheartedness, or passing out;
- Severe headache, confusion, slurred speech, arm or leg weakness, trouble walking, loss of coordination, feeling unsteady, very stiff muscles, high fever, profuse sweating, or tremors.
This document does not contain all possible side effects and others may occur. Check with your physician for additional information about side effects.
Dosage for Vuity
The dose of Vuity is one drop instilled in each eye once daily. If more than one topical ophthalmic product is being used, the products should be administered at least 5 minutes apart.
Vuity In Children
Presbyopia does not occur in the pediatric population.
What Drugs, Substances, or Supplements Interact with Vuity?
Vuity may interact with other medicines.
Tell your doctor all medications and supplements you use.
Vuity During Pregnancy and Breastfeeding
Tell your doctor if you are pregnant or plan to become pregnant before using Vuity; it is unknown how it might affect a fetus. It is unknown if Vuity passes into breast milk. Consult your doctor before breastfeeding.
Additional Information
Our Vuity (pilocarpine hydrochloride ophthalmic solution) 1.25%, for Topical Ophthalmic Use Side Effects Drug Center provides a comprehensive view of available drug information on the potential side effects when taking this medication.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.

SLIDESHOW
Vuity Professional Information
PATIENT INFORMATION
Night Driving
VUITY may cause temporary dim or dark vision. Advise patients to exercise caution with night driving and when hazardous activities are undertaken in poor illumination. [see WARNINGS AND PRECAUTIONS]
Accommodative Spasm
Temporary problems when changing focus between near and distant objects may occur. Advise patients not to drive or use machinery if vision is not clear (e.g., blurred vision). [see WARNINGS AND PRECAUTIONS]
When To Seek Physician Advice
Advise patients to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss. [see WARNINGS AND PRECAUTIONS]
Contact Lens Wear
Contact lens should be removed prior to the instillation of VUITY. Wait 10 minutes after dosing before reinserting contact lenses. [see WARNINGS AND PRECAUTIONS]
Avoiding Contamination Of The Product
Do not touch dropper tip to any surface, as this may contaminate the contents. [see WARNINGS AND PRECAUTIONS]
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart.

QUESTION
© Vuity Patient Information is supplied by Cerner Multum, Inc. and Vuity Consumer information is supplied by First Databank, Inc., used under license and subject to their respective copyrights.
Health Solutions From Our Sponsors
- Penis Curved When Erect
- Could I have CAD?
- Treat Bent Fingers
- Treat HR+, HER2- MBC
- Tired of Dandruff?
- Life with Cancer
Pill Identifier Tool Quick, Easy, Pill Identification
Drug Interaction Tool Check Potential Drug Interactions
Pharmacy Locator Tool Including 24 Hour, Pharmacies